Which Acid Would Be the Best Buffer at Ph Example
Us choose a buffer that has the pH we want. PH pK a log1 pK a 0 pK a So choose conjugates with a pK a closest to our target pH.

Buffer Solution Ph Calculations Henderson Hasselbalch Equation Explained Chemistry Problems Youtub Teaching Chemistry Biochemistry Notes Science Chemistry
For example pure sulfuric acids buffering at its second pK is theoretically 057 Eqmol-pH and like any other acid goes down fast at pH values distant by more than one pH.
. In this case if the solution contained equal molar concentrations of both the acid and the salt it would have a pH of 476. Which acid should you use to get the best buffer solution. A steady rmpH is required for the proper functioning of many chemical and biological systems including our blood for reactions to take place.
Many chemical reaction are carried out a constant pH. That solution is the one with both sodium hydroxide and hydrochloric acid. All of the answer choices are examples of the biological significance of buffers.
The acetate buffer would be effective of the pH range from about 374 to 574. These solutions consist of a weak acid and a salt of a weak acid. However because of its high density roughly 1gmL at 20 C it will tend to concentrate in small volumes so could be useful in weakly acidic buffers where other.
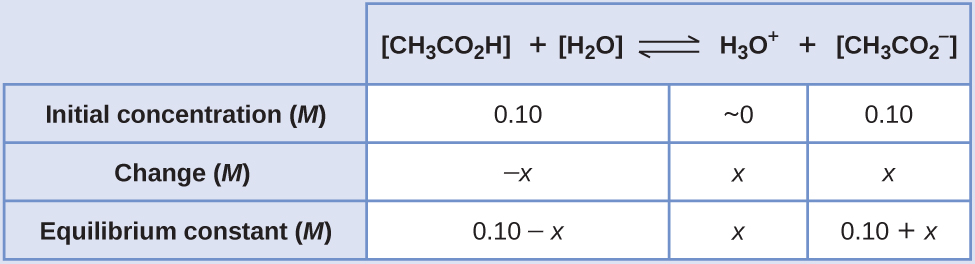
The number of moles of weak acid in the final buffer will be X Y for our example. A common example would be a mixture of ethanoic acid and sodium ethanoate in solution. How would you prepare a buffer solution of pH 2.
The ionization of weak acids results in an equilibrium system where the acid and its conjugate base coexist. In addition the pH of the acetate buffer was 399 whereas the calculated pH was 474. This is because our acidic buffer must include weak acid otherwise it wouldnt be an acidic buffer and wouldnt have any pH buffering function.
Buffers are generally good over the range pH pK a 1. Strong acids cannot buffer the pH of the solution. Buffers prevent acidic or basic foods from altering the pH of the digestive system.
Which of the four solutions tested is the best buffer against changes in pH caused by the addition of acid or base. Which is the best acid to use to make a buffer solution with a pH 20. Calculate the pH of the buffer prepared earlier 1000 mL of 010 M phosphate buffer at pH 740 after the addition of 100 mL of 10 M HCl.
It is possible to get this and have a pH 20 only if we use chlorous acid since its pK a is close to the target pH we need. Hydrochloric acid on the pH of a buffer solution. To choose the best buffer solution.
The pH scale is often said to range from 0 to 14 and most solutions do fall within this range. This is similar to the buffer system that resists small changes in pH. The Buffering Capacity of a Phosphate Buffer Lab Results 1.
The HCl will react with the conjugate base in the buffer HPO 4 2. Buffers prevent even the slightest changes in pH that can inhibit important biological molecules such as enzymes. The pKa of carbonic acid is 635 so it buffers better at pH 6 than at pH 7.
To ensure high capacity we need HA A-. The pKA closest to the middle of 4 and 6 so want as close to 5 is acetic acid at 47. PK60 and cysteine sulfhydryl.
The first solution has more buffer capacity because it contains more acetic acid and acetate ion. Buffers prevent stomach acids from escaping the. The ammonia buffer would be.
Acetic acid pK a 47 Explanation. The ammonia buffer would be effective between pH 824 1024. PH pK a logconj.
All the base will be neutralised in the reaction leaving some of the weak acid in the final buffer solution. Ka of acetic acid is 17 x 10-5. Acetic acid pK a 474chlorousacidpK a 195 or formic acid pK a 374.
Basic buffer has a basic pH and is prepared by mixing a weak base and its salt with strong acid. Acidic buffer solutions are commonly made from a weak acid and one of its salts - often a sodium salt. The best choice would be a buffer of H2PO4 and HPO42 because the pKa for this conjugate acidbase pair is close to the target pH of 70.
Buffers are generally good over the range pH pKa 1. The best buffer was the solution that had the least amount of change. For example the actual pH of acetic acid was 27 while the calculated pH was 288.
A weak acidbase best buffers about 1 pH point above and below its pKa. For example 1 L of a solution that is 10 M in acetic acid and 10 M in sodium acetate has a greater buffer capacity than 1 L of a solution that is 010 M in acetic acid and 010 M in sodium acetate even though both solutions have the same pH. An ICE chart is useful in determining the pH of the system after a strong acid has been added.
Up to 10 cash back Carbonic acid pK a 63 Uric acid pK a 39 Formic acid pK a 37 Correct answer. For example the bicarbonate buffering system is used to regulate the pH of blood and bicarbonate also. What is the pH range of a buffer.
In nature there are many systems that use buffering for pH regulation. Buffers are required in such systems in order to maintain a consistent rmpH An aqueous solution comprising a weak acid and its conjugate base or a weak base and its conjugate acid is. The pH scale is used to rank solutions in terms of acidity or basicity alkalinity.
Which amino acid is a buffer. Buffer solutions are characterized by a working pH range and capacity which indicates how much acid or base it can neutralize. You need a buffer with pH 45 and have four acids and their sodium salt availible.
What is the effective pH range of a buffer. An example of an acidic buffer solution is a mixture of sodium acetate and acetic acid pH 475. Since the scale is based on pH values it is logarithmic meaning that a change of 1 pH unit corresponds to a ten-fold change in H ion concentration.
For instance baby lotions that prevent rash and the growth of bacteria are buffered to keep the pH of 6. Hydrochloric Acid-Potassium Chloride Buffer 01. Acid With equal amounts of conjugate acid and base preferred so buffers can resist base and acid equally then.
The only amino acids with R-groups that have buffering capacity in the physiological pH range are histidine imidazole. However in the ammonium buffer system the pH of the buffer after adding 1 ml of hydrochloric acid and 1 ml of sodium hydroxide were higher than the computed values. Can all amino acids act as buffers.
500 mL of 0100 M HCl was added to a buffer consisting of 0025 moles of sodium acetate and 0030 moles of acetic acid. Outside of these ranges the solution can no longer resist changes in pH by added strong acids or bases. These buffer solutions are used to maintain basic conditions.
Buffers are widely used in everyday life. What is the pH of the buffer after the addition of the acid. For a more quantitative explanation see the other answers which introduce the Henderson Hasselbalch relationship and look up buffer capacity.
Buffer is also be defined as the solution of reserve acidity or alkalinity which resists change of pH upon the addition of small amount of acid or alkali.

Predicting The Ph Of A Buffer Ap Chemistry Chemistry Predictions
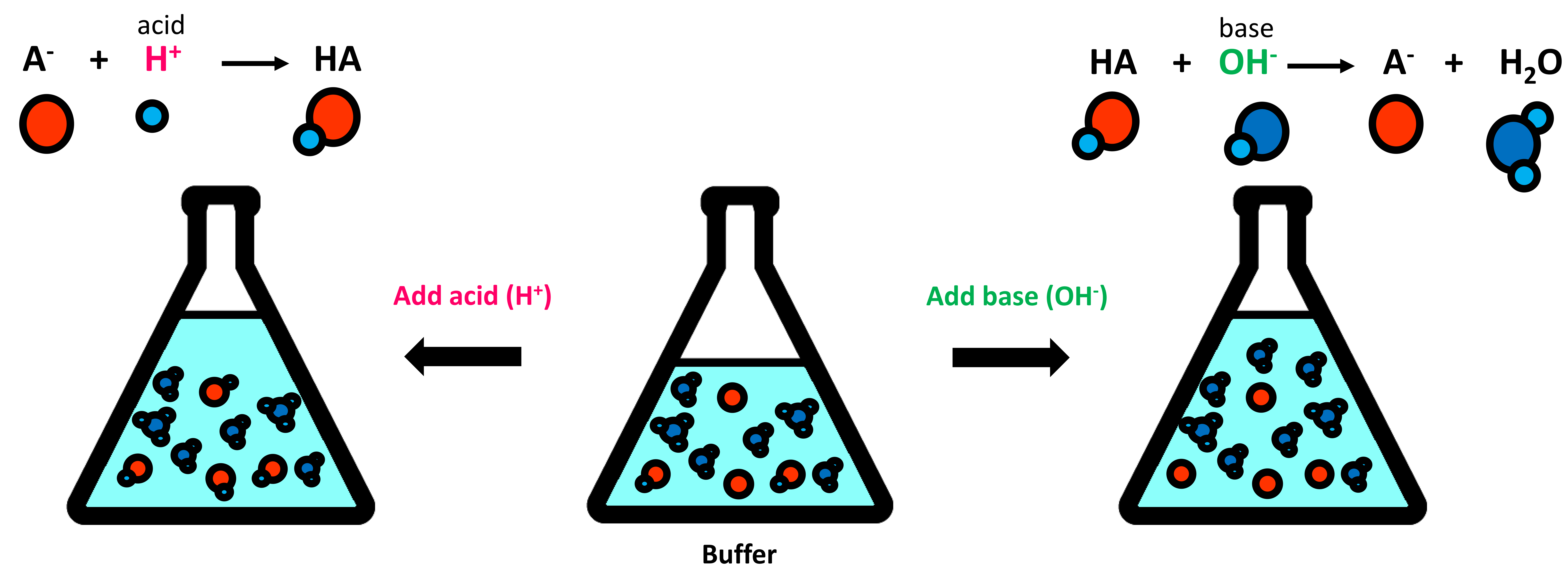
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

How To Calculate The Ph Of A Buffer Solution After Adding Acid Hcl Youtube

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy

Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Human Anatomy And Physiology
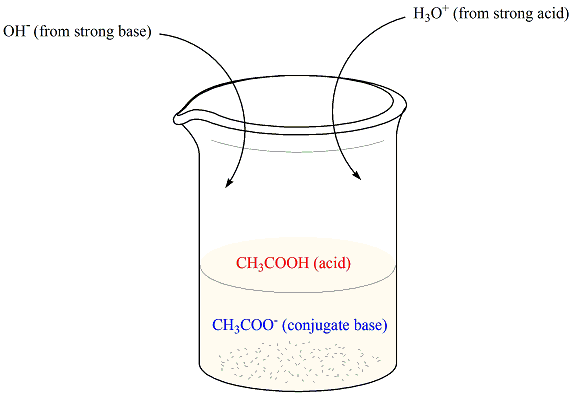
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy

Bicarbonate Buffer Systems Chemistry Lessons Systems Biology Nursing School Studying
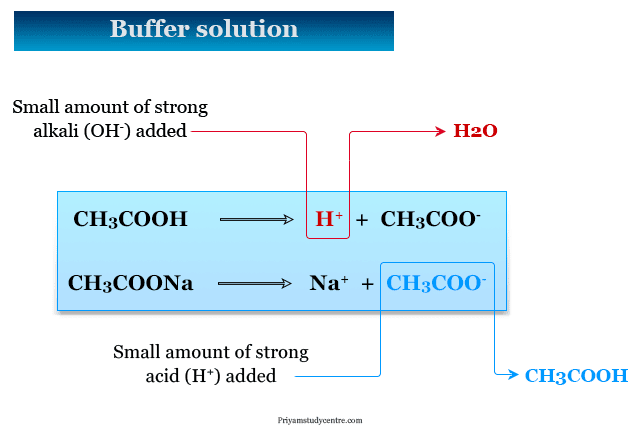
Buffer Solution Definition Types Uses

Buffer Chemistry Notes Chemistry Notes Biochemistry Notes Chemistry Classroom






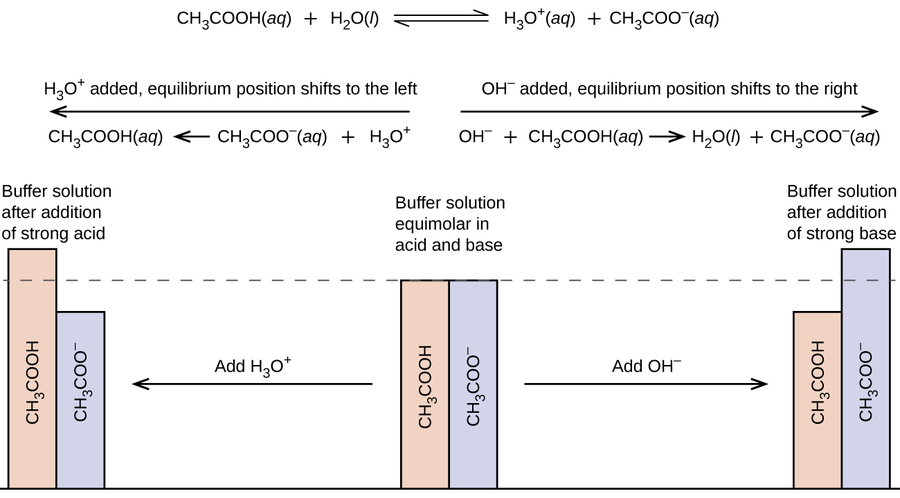
Comments
Post a Comment